The news wires for atrial fibrillation were abuzz this afternoon. The vigor and speed with which health news travels is striking.
Since 2.6 million Americans live with AF, my guess is that many are looking at the release of the Medtronic-sponsored TTOP-AF trial with anticipation. Here is a link to the press release. The trial purported to show benefits of Medtronic’s novel phased RF ablation system in treating persistent AF.
The study was small and released at a relatively small symposium in Venice Italy. The TTOP-AF trial randomized 210 patients with persistent AF (including flutter) to either ablation with Medtronic’s ablation system or conventional therapy with drugs and cardioversions.
They found, not surprisingly, that AF ablation reduced AF burden. AF ablation significantly reduced AF burden in 55.8% percent of patients versus only 26% of those treated with conventional medical treatment. Editorial comment: That kind of data is pretty typical.
The problem with the study comes in the safety arm: 21 of 138 patients in the ablation arm “experienced one or more protocol-defined adverse events.†This included four patients with manifest stroke, seven with PV stenosis and one death. Post-ablation MRI scans were not reported so we don’t know how many patients in each arm had non-symptomatic MRI brain lesions. (See below.)
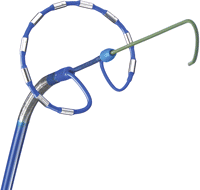
The still-investigational Medtronic ablation system used in the trial is much different than what is used in the real world. Their PVAC catheter is a novel circular catheter that can deliver simultaneous encircling lesion around the pulmonary veins. The notion is sweet because at the moment we have to deliver single lesions in a labor-intensive point-by-point manner. Placing a catheter just outside the orifice of the vein and making one burn would be great.
The problem with the technology rests in its physics. Energy delivery via the long electrodes on the PVAC catheter (which are not irrigated with saline, like our present catheter), increases the risk of clots forming in the left atrium. It has to do with varying temperatures at varying contact points along the left atrial wall-electrode(s) interface.
In considering the value of this novel new technology, it’s important to call your attention to this recent paper in the Journal of the American College of Cardiology. Here, the highly-respected AF researchers from Bordeaux showed an alarmingly high rate of embolic lesions (clot-related mini-strokes) in patients ablated with the very same PVAC catheter. In this trial, 37.5 % of patients ablated with the PVAC catheter had discrete lesions on MRI brain scans after the procedure.
I’ve said it before, and it bears repeating:
One of the major tenets of treating AF is to avoid making the treatment worse than the disease.
I’m afraid this technology, in its current form, will not prove safe enough. The risk of stroke is just too high. I’m sure Medtronic is working on making a safer catheter. Hopefully they will succeed.
JMM
3 replies on “AF ablation news: Don’t get too excited by press releases”
Am I reading this right? The PVAC catheter is no more effective and has a much higher rate of complications than other catheters in use today. This sounds like an abject failure to me, yet the press release hails it as a breakthrough.
It’s kind of a personal issue,” Dr John Hummel (Ohio State University Medical Center, Columbus), one of the TTOP investigators, told heartwire. “At times in our lab, we’ll do five ablations a day. These patients with long-standing and persistent atrial fibrillation have lower success rates and much longer procedure times. Having used this system, it provides a way to effectively ablate persistent atrial fibrillation in a way that is time efficient. It doesn’t expose the patient to long procedure times, more radiation, or anesthesia. It allows us to get in and ablate the triggers and the substrate and get out in a safe manner. That’s the crux of this trial.”
Silent ischemic event signal in previous studies
Earlier this year, two reports cast a bit of a shadow over the ablation system when researchers published data suggesting a higher rate of silent cerebral lesions in the multielectrode radiofrequency ablation catheter-treated patients compared with conventionally irrigated catheters [1,2]. Dr Claudia Herrera Siklódy (Herz-Zentrum, Bad Krozingen, Germany) and colleagues reported that postprocedure MRI revealed a new embolic lesion in 37.5% of patients treated with the multielectrode RF ablation catheter, compared with 7.4% of patients treated with a conventional irrigated catheter and 4.3% of patients treated with the cryoballoon ablation catheter.
Similarly, Dr Fiorenzo Gaita (University of Turin, Italy) and colleagues found that the incidence of silent cerebral lesions assessed by MRI was 38.9% in patients treated with the multielectrode RF ablation catheter. Comparatively, the incidence of silent cerebral lesions in patients treated with a conventional irrigated RF catheter (Navistar Thermocool, Biosense Webster) was 8.3% and 5.6% in patients treated with the cryoballoon catheter (Arctic Front, Medtronic).
At the time those studies were published, the investigators, as well as others, said there is little known about the clinical implications of silent cerebral embolization. To heartwire, Hummel said these silent ischemic events are not stroke events and resolve over time, although he expects the issue to be discussed during the upcoming FDA advisory panel. Data from irrigated catheters suggest microembolization occurs in anywhere from 6% to 37% of ablation procedures, but cognitive testing has shown that these lesions do not result in adverse outcomes, he added. heartwire will report on the October 27, 2011 FDA advisory panel, with updates throughout the day available on Twitter.